REXULTI® (Brexpiprazole) FDA Approved for Treating Agitation in Alzheimer's Disease Patients
Por um escritor misterioso
Last updated 04 julho 2024

REXULTI® (brexpiprazole) has been approved by the FDA for treating agitation associated with dementia due to Alzheimer's disease, making it the first and only pharmacological treatment for this indication in the US.

FDA Approves First Drug for Alzheimer's-Related Agitation Symptoms

FDA Approves Brexpiprazole for Agitation Associated with Alzheimer

Otsuka and Lundbeck Announce U.S. Food and Drug Administration

FDA Accepts sNDA for Rexulti for Alzheimer's Agitation

Ask the Pharmacist: New drug treats Alzheimer's agitation

Pyrls on Instagram: Brexpiprazole's (REXULTI) new indication

JAMA Neurology Publishes Complete Results of Positive Phase 3

FDA approves first drug to treat agitation linked with Alzheimer's

Alarm as FDA fast-tracks first antipsychotic drug for agitation in

Brexpiprazole (Rexulti) Approved for Alzheimer's Agitation
Recomendado para você
-
Rexulti Full Prescribing Information, Dosage & Side Effects04 julho 2024
-
 Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy04 julho 2024
Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy04 julho 2024 -
What you need to know about Rexulti (Brexpiprazole)04 julho 2024
-
REXULTI® (brexpiprazole), MDD04 julho 2024
-
 Why REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease04 julho 2024
Why REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease04 julho 2024 -
 Brexpiprazole Warnings: Side Effects of Rexulti04 julho 2024
Brexpiprazole Warnings: Side Effects of Rexulti04 julho 2024 -
 Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD04 julho 2024
Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD04 julho 2024 -
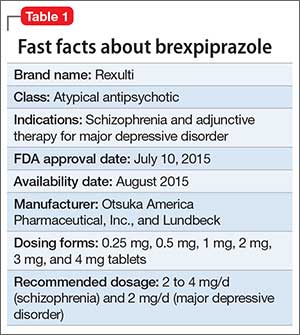 Brexpiprazole for schizophrenia and as adjunct for major depressive disorder04 julho 2024
Brexpiprazole for schizophrenia and as adjunct for major depressive disorder04 julho 2024 -
 Lundbeck Rexulti 0.5 Mg 7 Tabletas04 julho 2024
Lundbeck Rexulti 0.5 Mg 7 Tabletas04 julho 2024 -
 REXULTI (BREXPIPRAZOL) - NÃO TOME SEM ASSISTIR04 julho 2024
REXULTI (BREXPIPRAZOL) - NÃO TOME SEM ASSISTIR04 julho 2024
você pode gostar
-
 Jogo Pinte Sonic E Tails No Jogos 360 Coloring pages, Christmas window painting, Window painting04 julho 2024
Jogo Pinte Sonic E Tails No Jogos 360 Coloring pages, Christmas window painting, Window painting04 julho 2024 -
 Jogo Uno Stacko Torre De Empilhar - Mattel04 julho 2024
Jogo Uno Stacko Torre De Empilhar - Mattel04 julho 2024 -
 Wooden Gate Installation Chicagoland04 julho 2024
Wooden Gate Installation Chicagoland04 julho 2024 -
 Evil Dead: The Game é anunciado para 2021! - EvilHazard04 julho 2024
Evil Dead: The Game é anunciado para 2021! - EvilHazard04 julho 2024 -
 Dragon Ball Z Goku Drip Jacket - Male / Parachute / XS in 202304 julho 2024
Dragon Ball Z Goku Drip Jacket - Male / Parachute / XS in 202304 julho 2024 -
 Adore traz uma carismática aventura de captura de monstros04 julho 2024
Adore traz uma carismática aventura de captura de monstros04 julho 2024 -
 Big update! New tables, memberships, elite shop and more coming04 julho 2024
Big update! New tables, memberships, elite shop and more coming04 julho 2024 -
 Jesse Rutherford Talks New Solo Music Without The Neighbourhood – Billboard04 julho 2024
Jesse Rutherford Talks New Solo Music Without The Neighbourhood – Billboard04 julho 2024 -
 The Chosen Ones by Stratovarius (CD, Nov-1999, Noise) for sale online04 julho 2024
The Chosen Ones by Stratovarius (CD, Nov-1999, Noise) for sale online04 julho 2024 -
 Broken Bow Golf TournAment - Rotary District 563004 julho 2024
Broken Bow Golf TournAment - Rotary District 563004 julho 2024
