Transcriptional patterns of sexual dimorphism and in host developmental programs in the model parasitic nematode Heligmosomoides bakeri, Parasites & Vectors
Por um escritor misterioso
Last updated 01 junho 2024
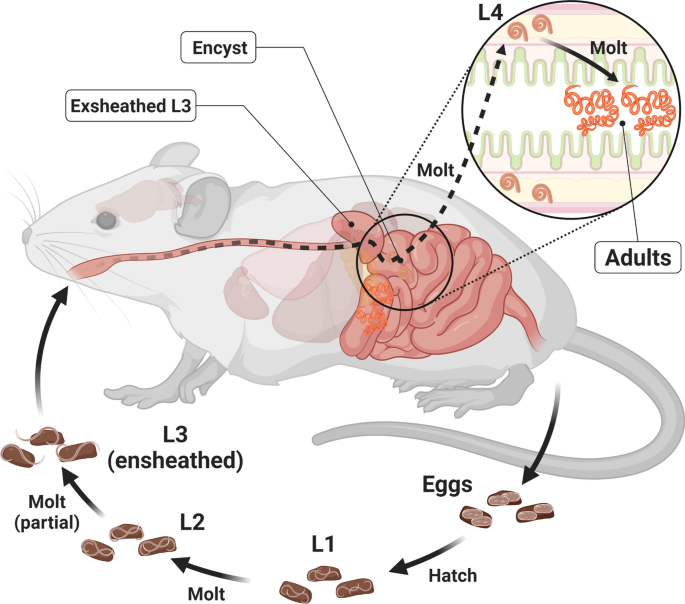
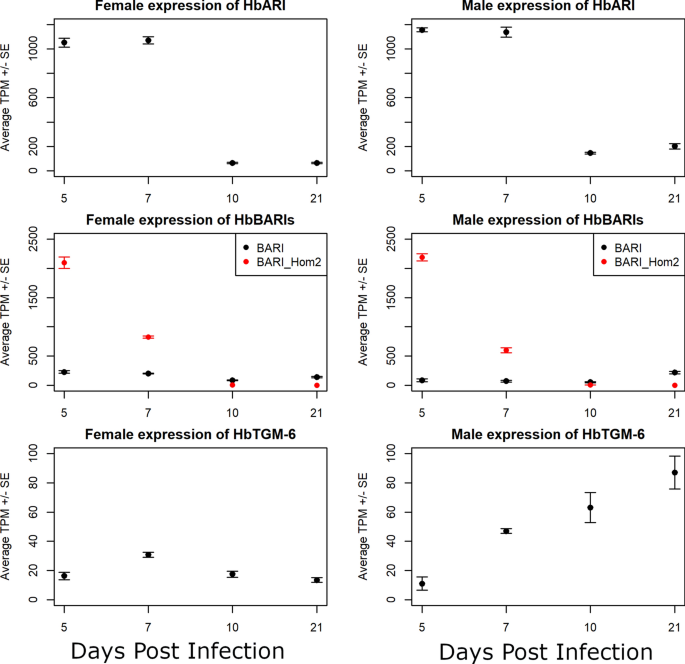
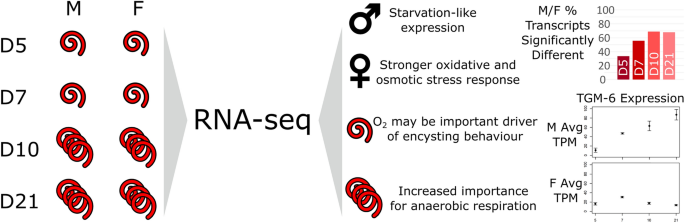
Background Heligmosomoides bakeri (often mistaken for Heligmosomoides polygyrus) is a promising model for parasitic nematodes with the key advantage of being amenable to study and manipulation within a controlled laboratory environment. While draft genome sequences are available for this worm, which allow for comparative genomic analyses between nematodes, there is a notable lack of information on its gene expression. Methods We generated biologically replicated RNA-seq datasets from samples taken throughout the parasitic life of H. bakeri. RNA from tissue-dwelling and lumen-dwelling worms, collected under a dissection microscope, was sequenced on an Illumina platform. Results We find extensive transcriptional sexual dimorphism throughout the fourth larval and adult stages of this parasite and identify alternative splicing, glycosylation, and ubiquitination as particularly important processes for establishing and/or maintaining sex-specific gene expression in this species. We find sex-linked differences in transcription related to aging and oxidative and osmotic stress responses. We observe a starvation-like signature among transcripts whose expression is consistently upregulated in males, which may reflect a higher energy expenditure by male worms. We detect evidence of increased importance for anaerobic respiration among the adult worms, which coincides with the parasite’s migration into the physiologically hypoxic environment of the intestinal lumen. Furthermore, we hypothesize that oxygen concentration may be an important driver of the worms encysting in the intestinal mucosa as larvae, which not only fully exposes the worms to their host’s immune system but also shapes many of the interactions between the host and parasite. We find stage- and sex-specific variation in the expression of immunomodulatory genes and in anthelmintic targets. Conclusions We examine how different the male and female worms are at the molecular level and describe major developmental events that occur in the worm, which extend our understanding of the interactions between this parasite and its host. In addition to generating new hypotheses for follow-up experiments into the worm’s behavior, physiology, and metabolism, our datasets enable future more in-depth comparisons between nematodes to better define the utility of H. bakeri as a model for parasitic nematodes in general. Graphical Abstract

The production of excretory-secretory molecules from

PDF) Small RNAs in parasitic nematodes - forms and functions
Transcriptional patterns of sexual dimorphism and in host

PDF) The production of excretory-secretory molecules from

PDF) TGF-β mimic proteins form an extended gene family in the
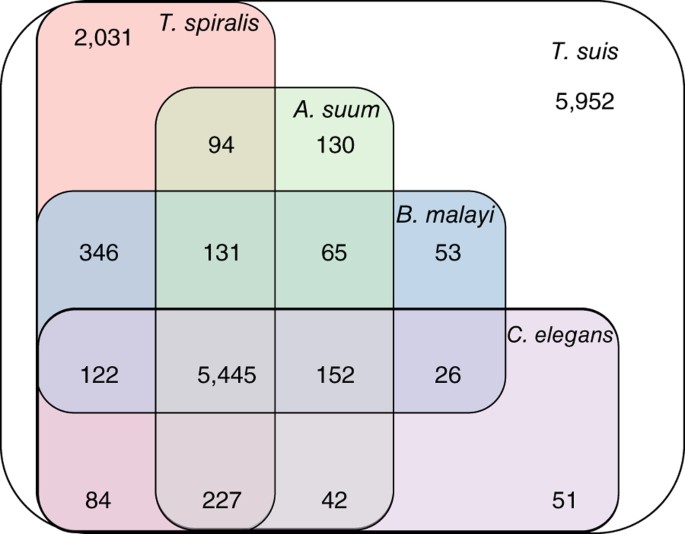
Genome and transcriptome of the porcine whipworm Trichuris suis
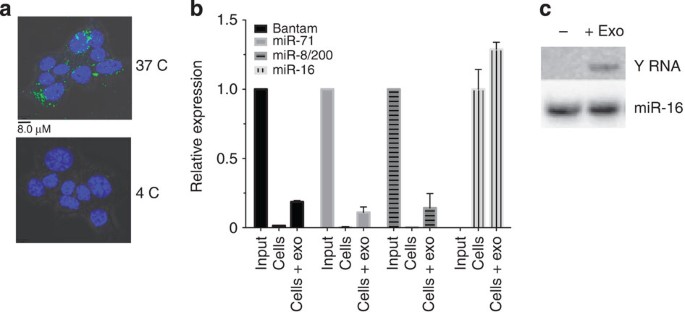
Exosomes secreted by nematode parasites transfer small RNAs to

TGF-β mimic proteins form an extended gene family in the murine
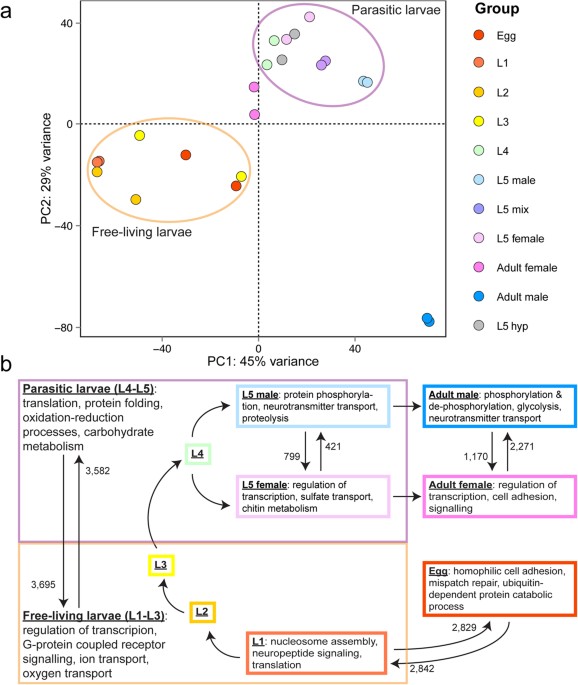
Transcriptional patterns of sexual dimorphism and in host
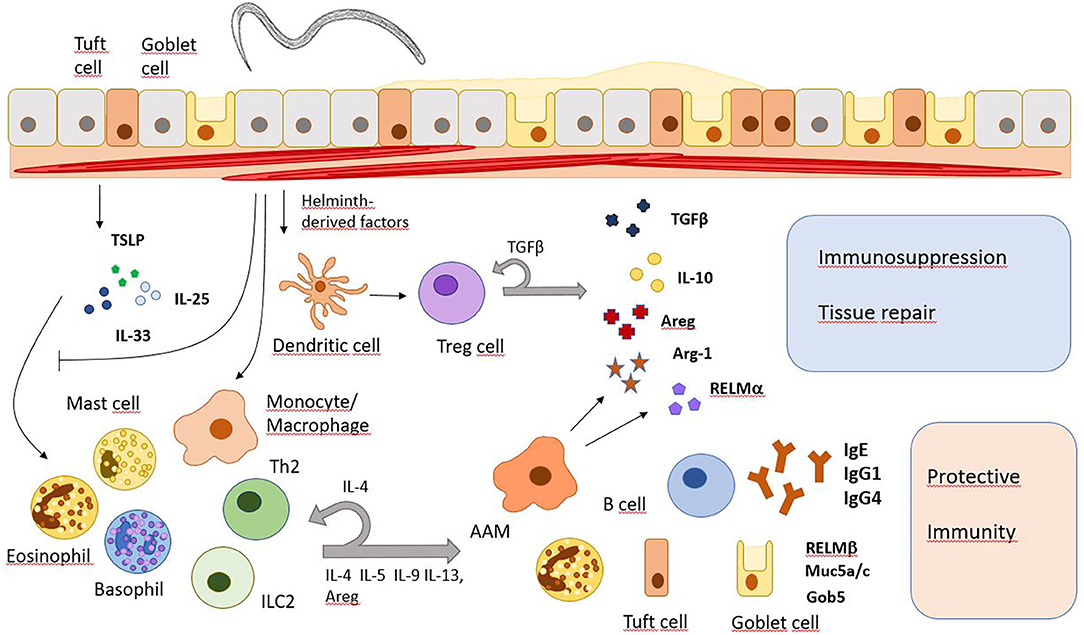
Frontiers Immunomodulation and Immune Escape Strategies of

The production of excretory-secretory molecules from
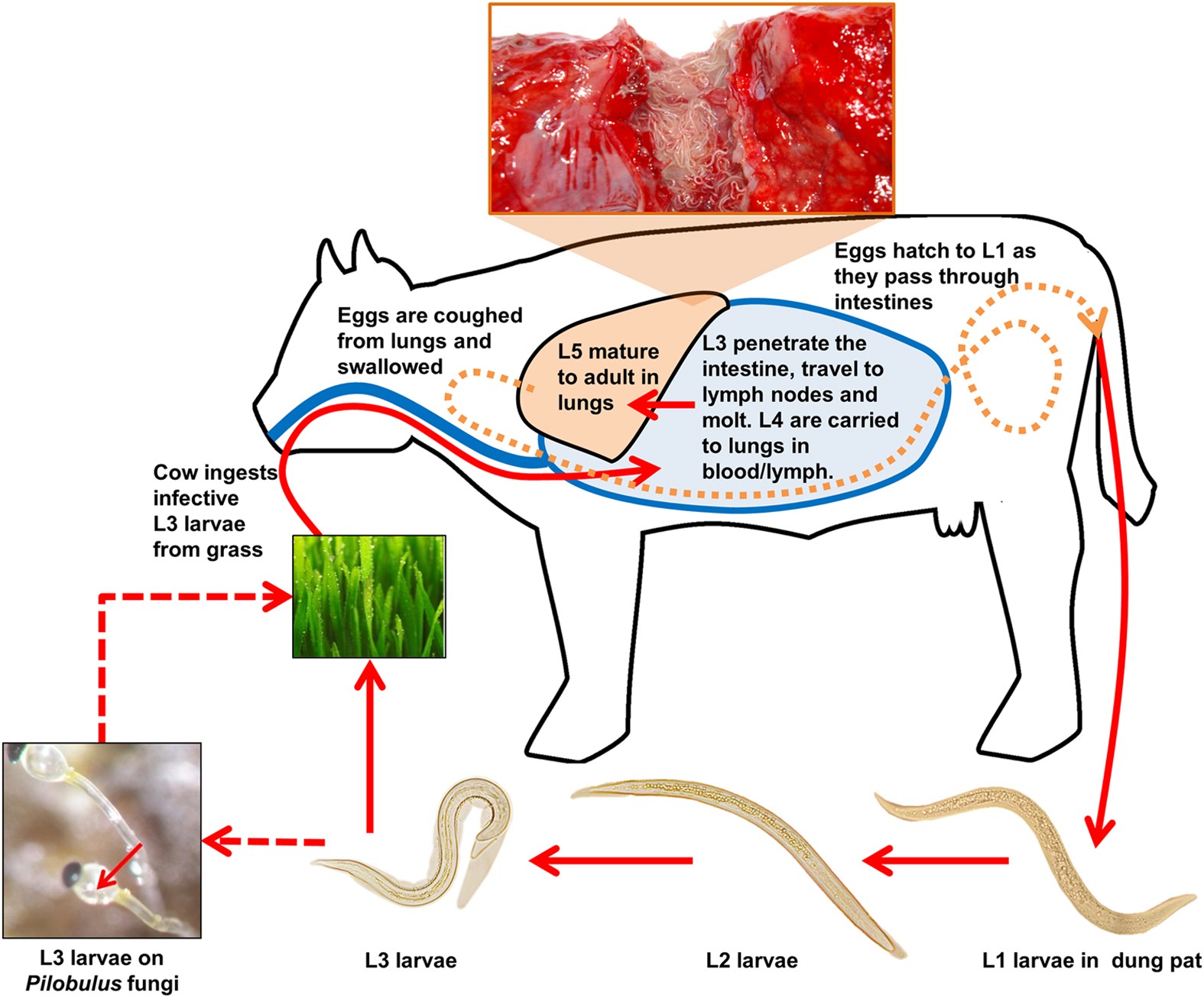
Dictyocaulus viviparus genome, variome and transcriptome elucidate

PDF) Immunity to the model intestinal helminth parasite
Dictyocaulus viviparus genome, variome and transcriptome
Recomendado para você
-
 Hamsters: Diet, habits & types01 junho 2024
Hamsters: Diet, habits & types01 junho 2024 -
 Winter white dwarf hamster - Wikipedia01 junho 2024
Winter white dwarf hamster - Wikipedia01 junho 2024 -
 Life cycle of S. mansoni , illustrating the collection points for in01 junho 2024
Life cycle of S. mansoni , illustrating the collection points for in01 junho 2024 -
 Laboratory maintenance of the bacterial endosymbiont, Neorickettsia sp., through the life cycle of a digenean, Plagiorchis elegans - ScienceDirect01 junho 2024
Laboratory maintenance of the bacterial endosymbiont, Neorickettsia sp., through the life cycle of a digenean, Plagiorchis elegans - ScienceDirect01 junho 2024 -
Histone methylation changes are required for life cycle progression in the human parasite Schistosoma mansoni01 junho 2024
-
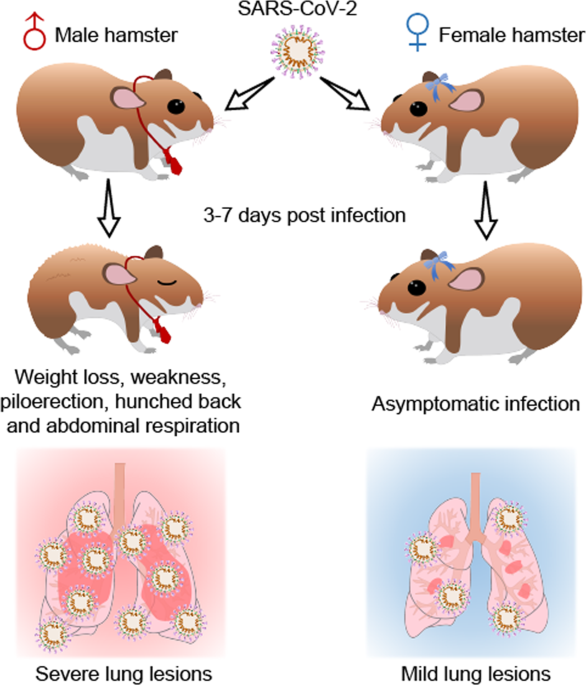 Gender associates with both susceptibility to infection and pathogenesis of SARS-CoV-2 in Syrian hamster01 junho 2024
Gender associates with both susceptibility to infection and pathogenesis of SARS-CoV-2 in Syrian hamster01 junho 2024 -
 Prolee Hamster Cage Wooden 32 Inch Mice and Rat Habitat Openable Top with Acrylic Sheets Solid Built : Pet Supplies01 junho 2024
Prolee Hamster Cage Wooden 32 Inch Mice and Rat Habitat Openable Top with Acrylic Sheets Solid Built : Pet Supplies01 junho 2024 -
 Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books01 junho 2024
Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books01 junho 2024 -
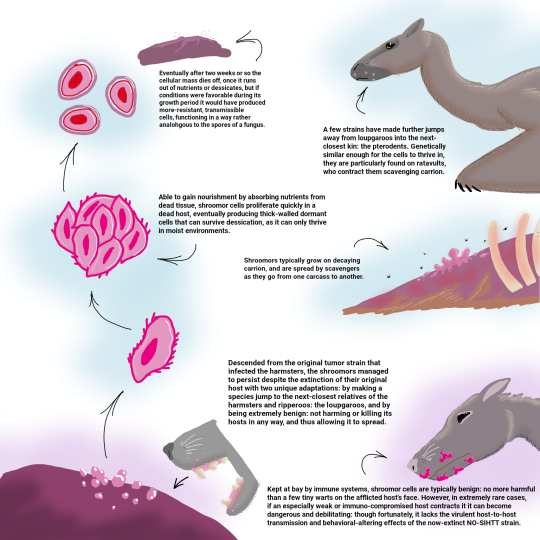 ceo of hamster evolution — The Early Temperocene: 140 million years01 junho 2024
ceo of hamster evolution — The Early Temperocene: 140 million years01 junho 2024 -
 HAMSTERS!!!!!01 junho 2024
HAMSTERS!!!!!01 junho 2024
você pode gostar
-
Coleção de jogos do doodle01 junho 2024
-
You Go Down Just Like Going Merry #manga #luffy #luffyonepiece #goi01 junho 2024
-
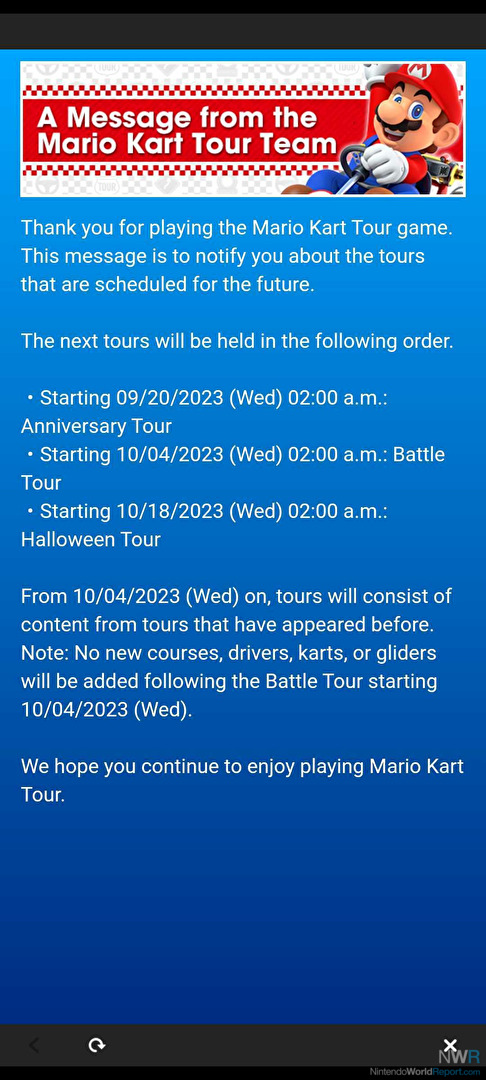 Mario Kart Tour To Enter Maintenance Mode In October - News01 junho 2024
Mario Kart Tour To Enter Maintenance Mode In October - News01 junho 2024 -
 Resident Evil HD Remaster - PCGamingWiki PCGW - bugs, fixes01 junho 2024
Resident Evil HD Remaster - PCGamingWiki PCGW - bugs, fixes01 junho 2024 -
 Anime Adventure: Unleash Your Creativity and Master Your Drawing Skills: A Coloring and Drawing Book for Anime Fans with Original Illustrations01 junho 2024
Anime Adventure: Unleash Your Creativity and Master Your Drawing Skills: A Coloring and Drawing Book for Anime Fans with Original Illustrations01 junho 2024 -
 Pizzeria Papa Luigi, Cam. Condesa, s/n in Fuengirola - Restaurant reviews01 junho 2024
Pizzeria Papa Luigi, Cam. Condesa, s/n in Fuengirola - Restaurant reviews01 junho 2024 -
 HOW TO CHANGE ROBLOX USERNAME FOR FREE WITHOUT PAYING 1,000 ROBUX!! (STILL WORKS)01 junho 2024
HOW TO CHANGE ROBLOX USERNAME FOR FREE WITHOUT PAYING 1,000 ROBUX!! (STILL WORKS)01 junho 2024 -
Infinite Dramas01 junho 2024
-
 kawaii para colorir 26736954 Foto de stock no Vecteezy01 junho 2024
kawaii para colorir 26736954 Foto de stock no Vecteezy01 junho 2024 -
 Gakuen Chaika! Manga Online Free - Manganelo01 junho 2024
Gakuen Chaika! Manga Online Free - Manganelo01 junho 2024

