Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial - The Lancet
Por um escritor misterioso
Last updated 28 junho 2024


Full article: New pharmacotherapeutic options for oral

Treatment Effect on Stroke Subtypes According to TOAST (Trial of

News at XI: moving beyond factor Xa inhibitors - ScienceDirect

Antithrombotic Therapy for Primary and Secondary Prevention of

Design and Preclinical Characterization Program toward Asundexian

Design and Preclinical Characterization Program toward Asundexian
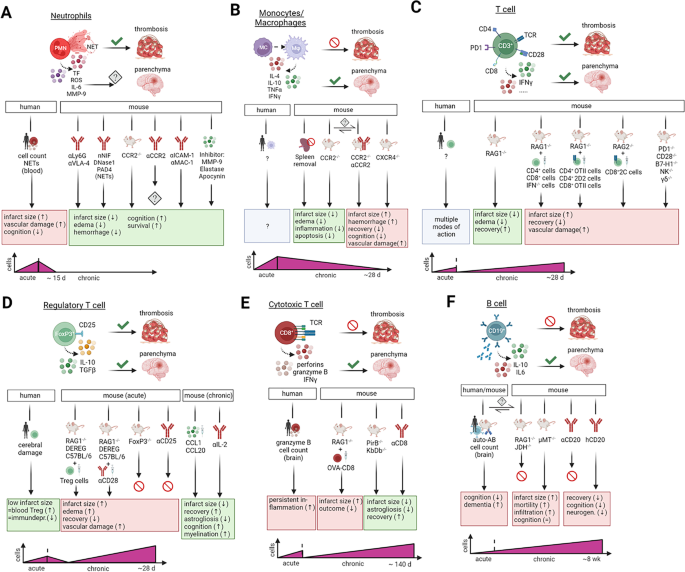
Thromboinflammatory challenges in stroke pathophysiology
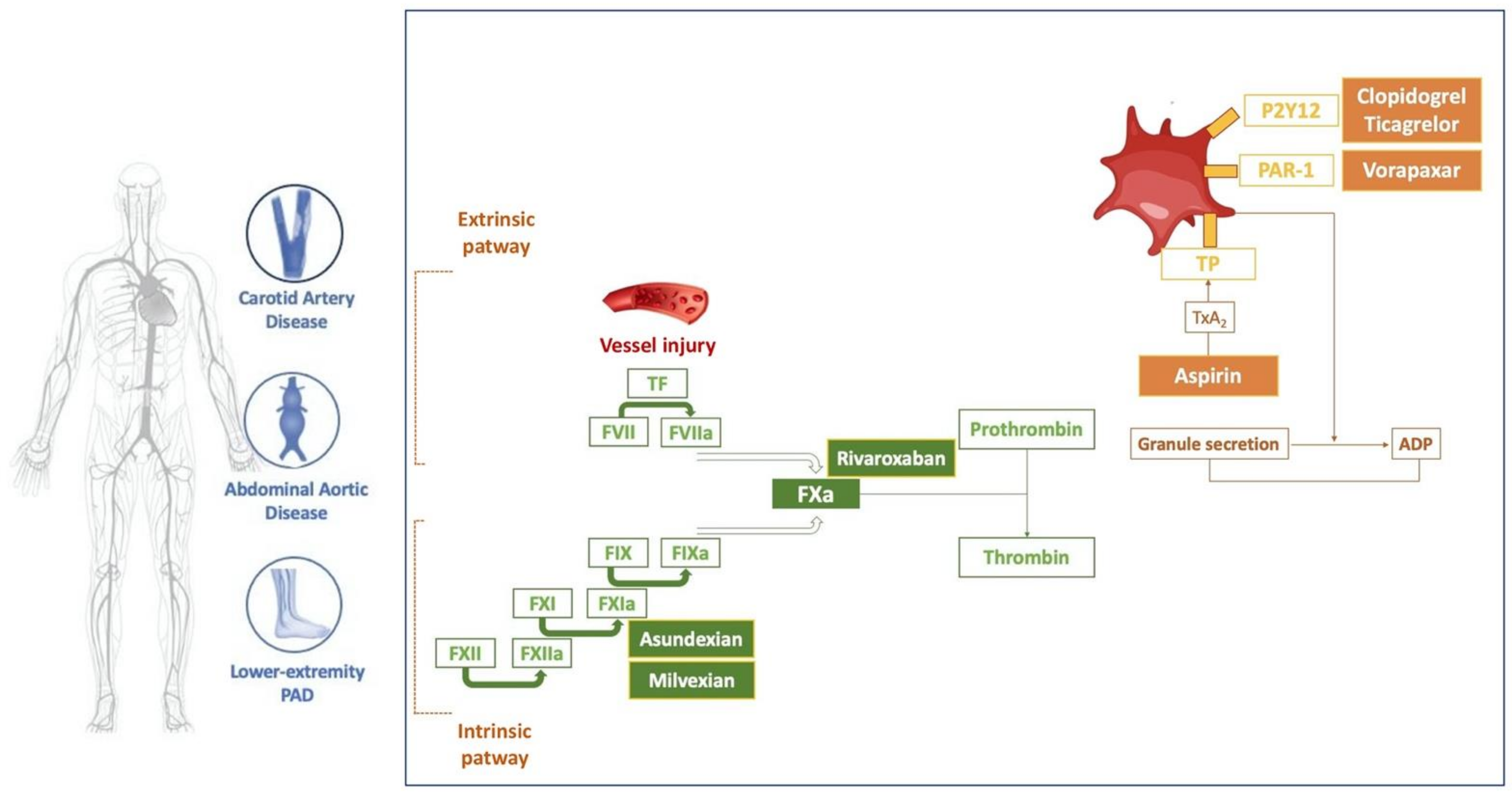
JCDD, Free Full-Text

Effects of Tablet Formulation, Food, or Gastric pH on the

PDF) Efficacy and Safety of Oral Factor XIa Inhibitors in Stroke

Ischaemic stroke despite antiplatelet therapy: Causes and outcomes

Dual Antiplatelet Therapies and Causes in Minor Stroke or
(BAY 2433334) is an orally active coagulation factor Xia (FXIa) inhibitor. Asundexian binds directly, potently, and reversibly to the active site
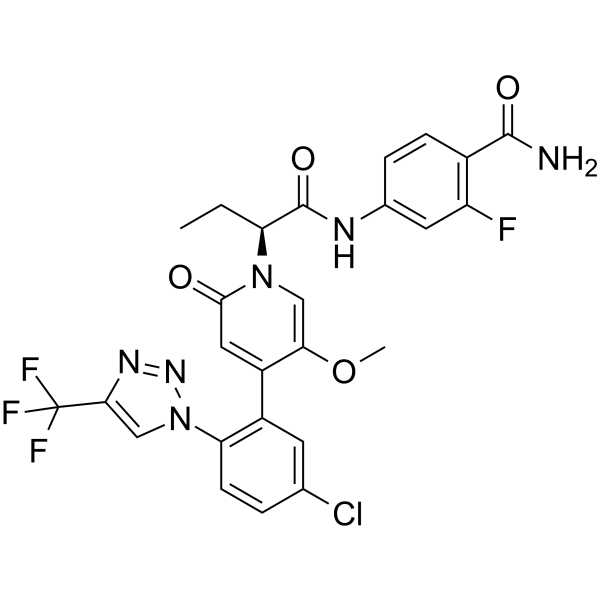
Asundexian

A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double
Recomendado para você
-
 Brain Test Level 372 Walkthrough28 junho 2024
Brain Test Level 372 Walkthrough28 junho 2024 -
 LARGE PRINT FUN & EASY BRAIN WORKOUTS FOR SENIORS: Volume 5: 6 in 1 Senior Living28 junho 2024
LARGE PRINT FUN & EASY BRAIN WORKOUTS FOR SENIORS: Volume 5: 6 in 1 Senior Living28 junho 2024 -
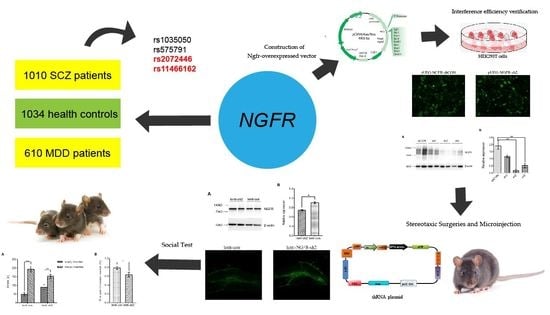 Brain Sciences, Free Full-Text28 junho 2024
Brain Sciences, Free Full-Text28 junho 2024 -
 DOP 2 Level 372 Answer the phone Answer - Daze Puzzle28 junho 2024
DOP 2 Level 372 Answer the phone Answer - Daze Puzzle28 junho 2024 -
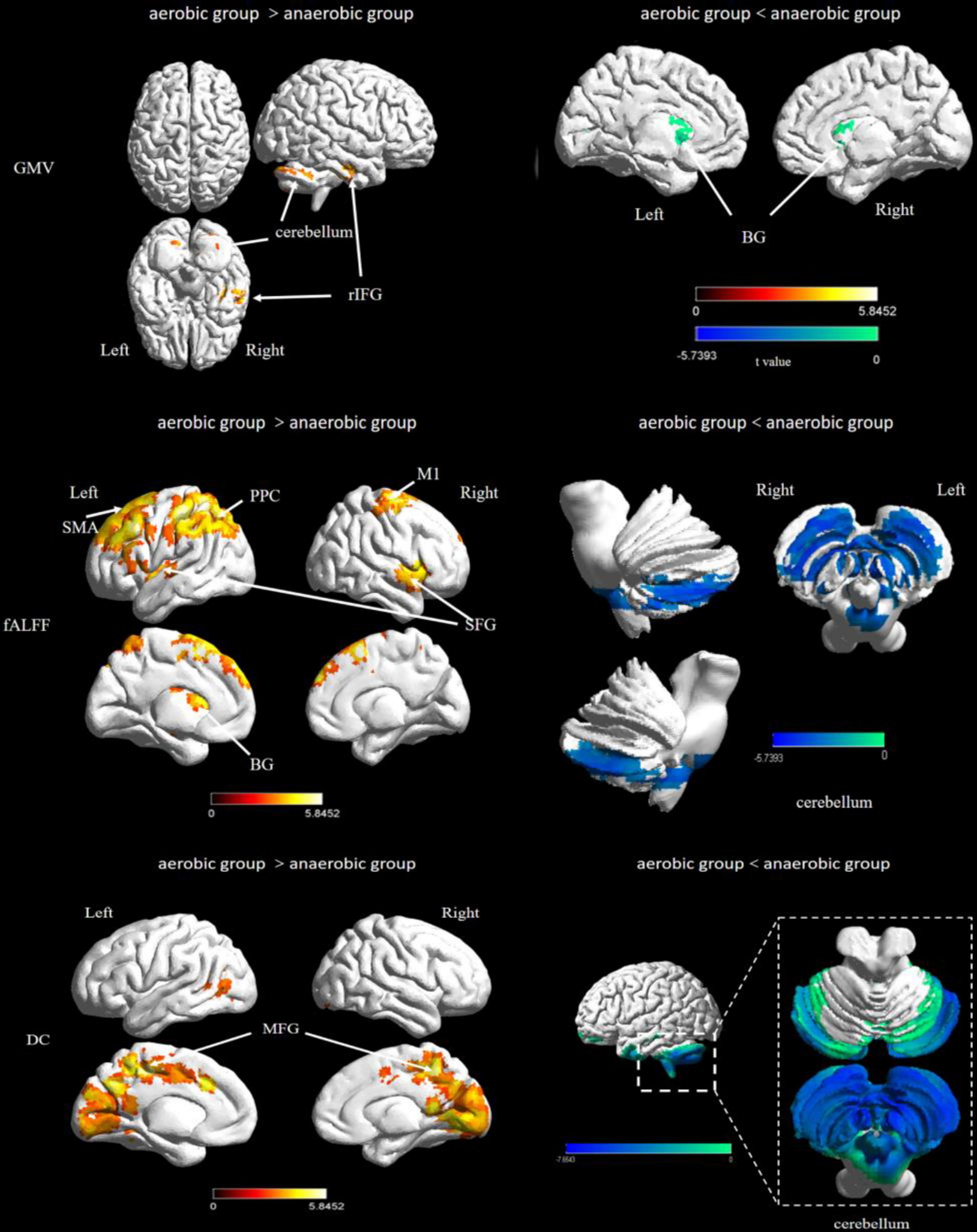 Frontiers Exercise Intensity and Brain Plasticity: What's the Difference of Brain Structural and Functional Plasticity Characteristics Between Elite Aerobic and Anaerobic Athletes?28 junho 2024
Frontiers Exercise Intensity and Brain Plasticity: What's the Difference of Brain Structural and Functional Plasticity Characteristics Between Elite Aerobic and Anaerobic Athletes?28 junho 2024 -
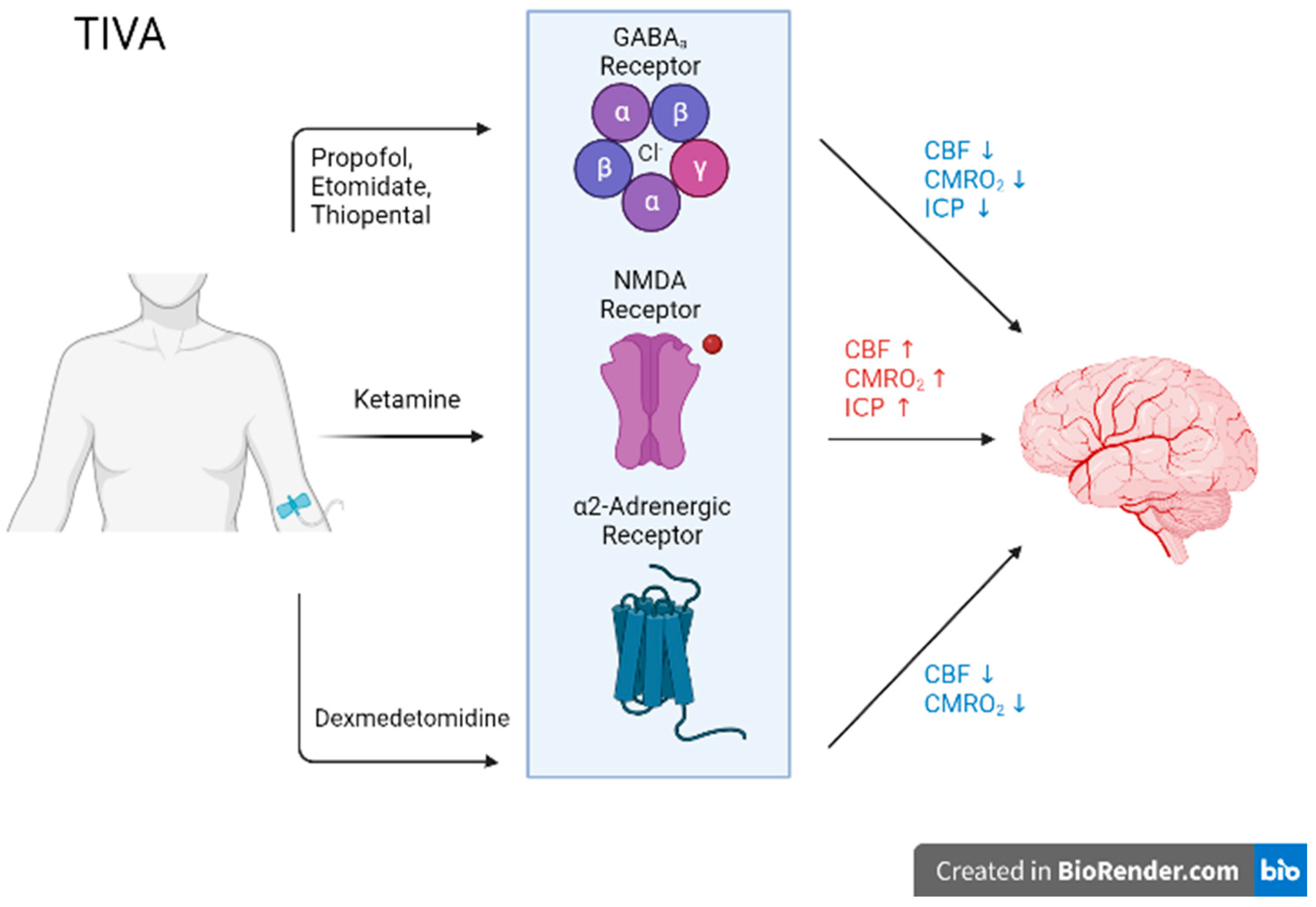 Biomedicines, Free Full-Text28 junho 2024
Biomedicines, Free Full-Text28 junho 2024 -
Introduction to Cortical Neurons28 junho 2024
-
 Integrated Workflow Intelligence28 junho 2024
Integrated Workflow Intelligence28 junho 2024 -
Quest Diagnostics launches Alzheimer's first direct-to-consumer blood test28 junho 2024
-
 Brain Test Level 372 He wants big muscles28 junho 2024
Brain Test Level 372 He wants big muscles28 junho 2024
você pode gostar
-
 Break Point: that's when the last episodes of the first season will be released28 junho 2024
Break Point: that's when the last episodes of the first season will be released28 junho 2024 -
 How To Create a Minecraft Server on Ubuntu 22.0428 junho 2024
How To Create a Minecraft Server on Ubuntu 22.0428 junho 2024 -
 Parceria entre Clube Agro Brasil e Agrofy amplia vitrine do varejo agro – Revista Ideal28 junho 2024
Parceria entre Clube Agro Brasil e Agrofy amplia vitrine do varejo agro – Revista Ideal28 junho 2024 -
 Dragon Ball Super: Manga Chapter 88 - Official Discussion Thread - Page 6 • Kanzenshuu28 junho 2024
Dragon Ball Super: Manga Chapter 88 - Official Discussion Thread - Page 6 • Kanzenshuu28 junho 2024 -
 IMDB adversarial examples by attacking Word CNN. Blue and red texts28 junho 2024
IMDB adversarial examples by attacking Word CNN. Blue and red texts28 junho 2024 -
 Naruto Shippuden camiseta de manga curta dos homens, Anti-Village Akatsuki Nuvem, Front Back 3D Impresso - AliExpress28 junho 2024
Naruto Shippuden camiseta de manga curta dos homens, Anti-Village Akatsuki Nuvem, Front Back 3D Impresso - AliExpress28 junho 2024 -
 Articles Archive » All Chess Posts » Chess Intellect28 junho 2024
Articles Archive » All Chess Posts » Chess Intellect28 junho 2024 -
 CAR DEALERSHIP TYCOON CODES NEW UPDATE ROBLOX28 junho 2024
CAR DEALERSHIP TYCOON CODES NEW UPDATE ROBLOX28 junho 2024 -
 Vem aí a segunda temporada da série sobre 3 Mulheres portuguesas emblemáticas – Stars Online28 junho 2024
Vem aí a segunda temporada da série sobre 3 Mulheres portuguesas emblemáticas – Stars Online28 junho 2024 -
Rosenfield - 💜𝕰𝖘𝖙𝖆𝖗𝖔𝖘𝖘𝖆 𝕺 𝖆𝖓𝖏𝖔 𝖈𝖆í𝖉𝖔28 junho 2024