Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Last updated 17 junho 2024
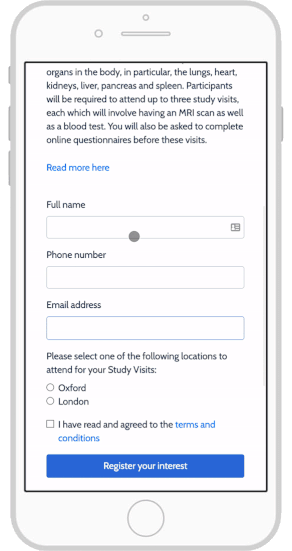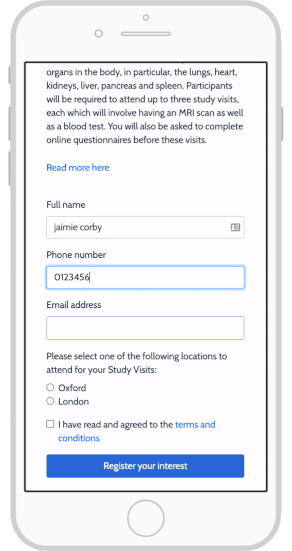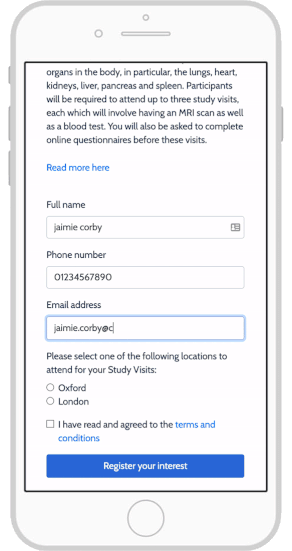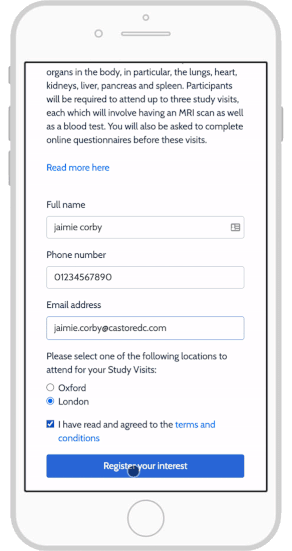

eConsent in Study Runner - OpenClinica Reference Guide

Patient Centric Clinical Trials and Use of eConsent, ePRO & eCOA

E-Consent for Clinical Trials - Best Practices & Common Mistakes

7 ways eConsent can help health researchers achieve better

Top 4 eConsent Questions in Clinical Research: Forms & More

Using REDCap for eConsent – VICTR – Vanderbilt Institute for
eConsent In Healthcare Market Demand and Future Scope Analysis

Electronic Consent Form Template - Fill Online, Printable
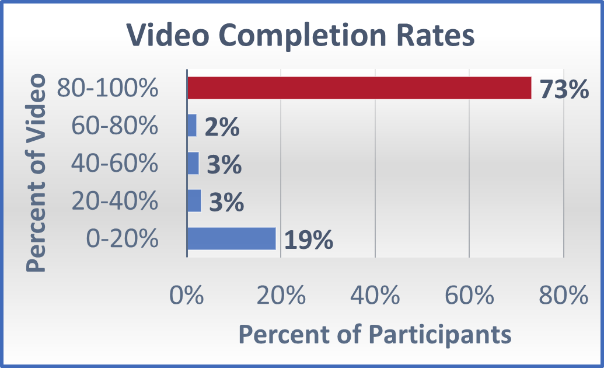
eConsent In Clinical Trials Insights For Implementation During

Using e-Consent Forms in Your Clinical Research

Econsent for Clinical Trials Electronic Informed Consent for
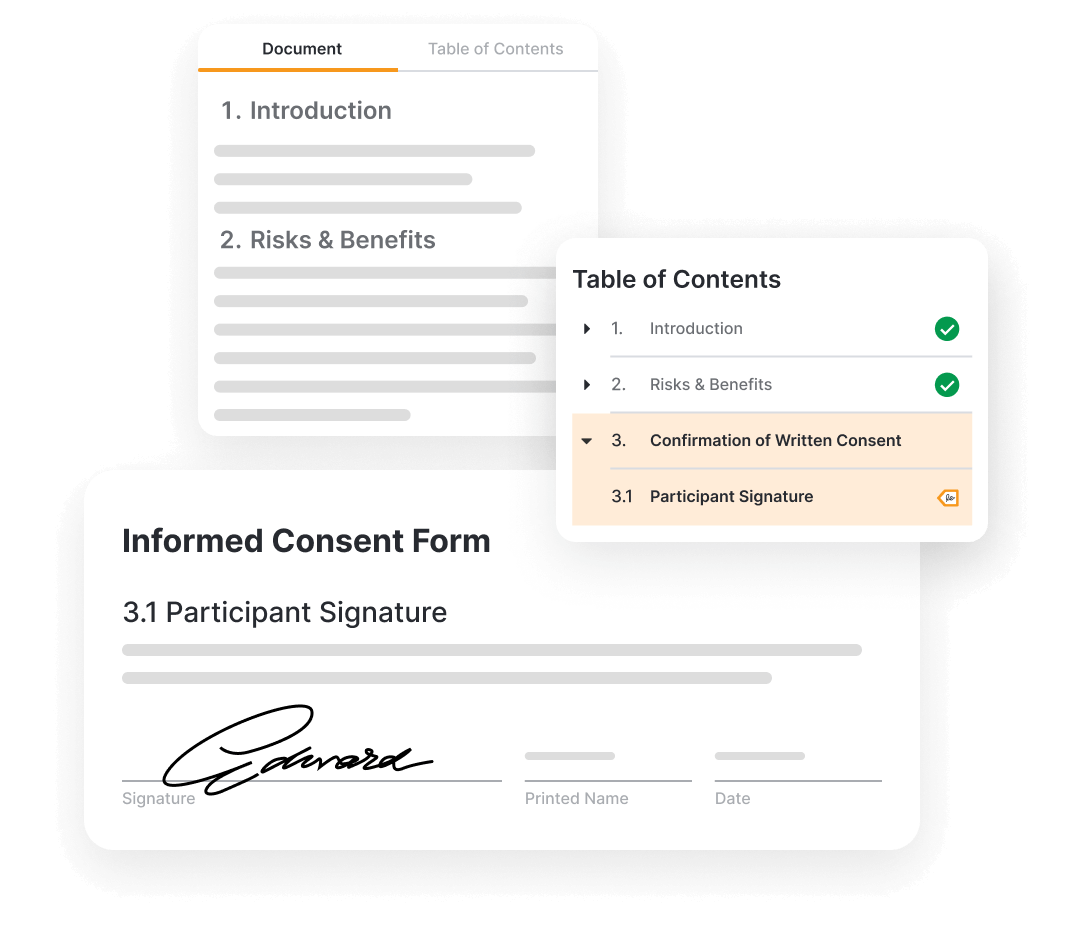
Veeva eConsent, End-to-End eConsent Platform
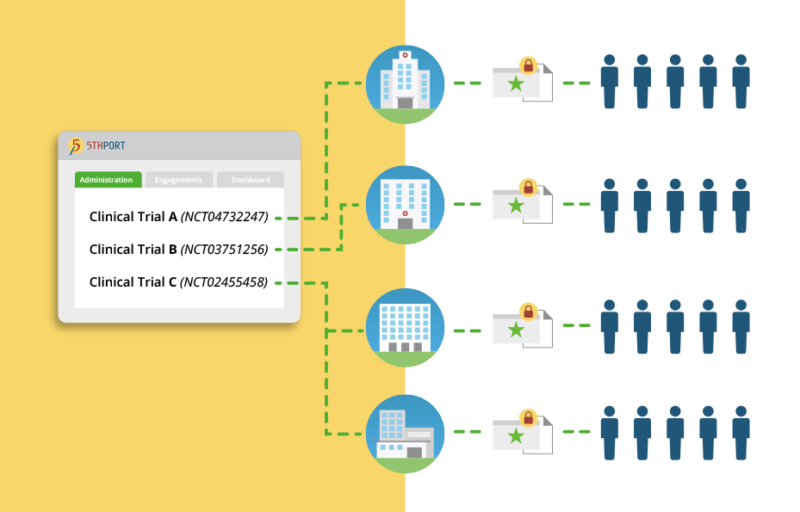
Clinical Solution, eConsent for Clinical Trials
Recomendado para você
-
 Free Online Image Editor Love gif, Dream pictures, I love you honey17 junho 2024
Free Online Image Editor Love gif, Dream pictures, I love you honey17 junho 2024 -
 Opening heart locket gif maker - Erase BG17 junho 2024
Opening heart locket gif maker - Erase BG17 junho 2024 -
 Bringing the Spotify Heart to Life17 junho 2024
Bringing the Spotify Heart to Life17 junho 2024 -
 Add glitters to images Free Online Image Editor17 junho 2024
Add glitters to images Free Online Image Editor17 junho 2024 -
 Your A.I. Companion Will Support You No Matter What17 junho 2024
Your A.I. Companion Will Support You No Matter What17 junho 2024 -
 Online Banner Maker - Badge with Centered Text by Placeit on Dribbble17 junho 2024
Online Banner Maker - Badge with Centered Text by Placeit on Dribbble17 junho 2024 -
 Welcome: Red Heart17 junho 2024
Welcome: Red Heart17 junho 2024 -
 Gameplay Enhancements news - Nin Online - Indie DB17 junho 2024
Gameplay Enhancements news - Nin Online - Indie DB17 junho 2024 -
 Virtual and Online Love Letter - Virtual Gifts at17 junho 2024
Virtual and Online Love Letter - Virtual Gifts at17 junho 2024 -
 Electrocardiography - Wikipedia17 junho 2024
Electrocardiography - Wikipedia17 junho 2024
você pode gostar
-
aranha scarlet dublado|Pesquisa do TikTok17 junho 2024
-
 Comparing & Combining EVERY LEGO Harry Potter Hogwarts Castle 2018-2020 vs 2021-202217 junho 2024
Comparing & Combining EVERY LEGO Harry Potter Hogwarts Castle 2018-2020 vs 2021-202217 junho 2024 -
 Kit com 2 Meia Calça 7/8 para Boneca Barbie * Branco Rosa17 junho 2024
Kit com 2 Meia Calça 7/8 para Boneca Barbie * Branco Rosa17 junho 2024 -
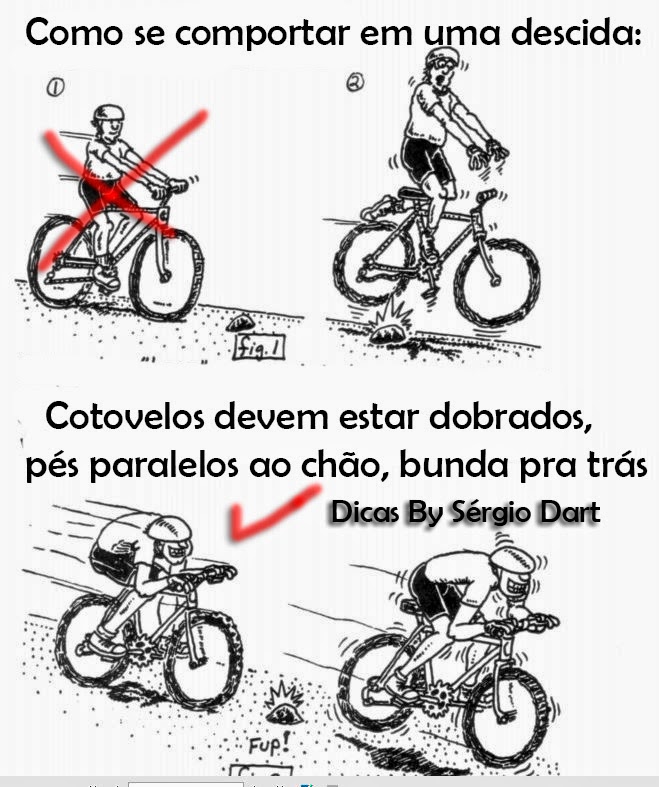 Dica de como se comportar na bike em uma descida17 junho 2024
Dica de como se comportar na bike em uma descida17 junho 2024 -
 Tribal Hunter, Made With GameMaker17 junho 2024
Tribal Hunter, Made With GameMaker17 junho 2024 -
 Hello Secret Neighbor APK for Android Download17 junho 2024
Hello Secret Neighbor APK for Android Download17 junho 2024 -
 OS MELHORES MEMES DE SÃO PAULO 2 X 1 CORINTHIANS SÃO PAULO ELIMINA CORINTHIANS PAULISTÃO 202217 junho 2024
OS MELHORES MEMES DE SÃO PAULO 2 X 1 CORINTHIANS SÃO PAULO ELIMINA CORINTHIANS PAULISTÃO 202217 junho 2024 -
 Men's Zombie Holocaust Rock N Roll Party Patrol Concert T Shirt XXL 2XL Gildan17 junho 2024
Men's Zombie Holocaust Rock N Roll Party Patrol Concert T Shirt XXL 2XL Gildan17 junho 2024 -
 Mommy Long Legs Gacha Life - Club 1 by JennyArt2 on DeviantArt17 junho 2024
Mommy Long Legs Gacha Life - Club 1 by JennyArt2 on DeviantArt17 junho 2024 -
 BRASIL 3 X 0 TAILÂNDIA, MELHORES MOMENTOS17 junho 2024
BRASIL 3 X 0 TAILÂNDIA, MELHORES MOMENTOS17 junho 2024
