Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Last updated 17 junho 2024
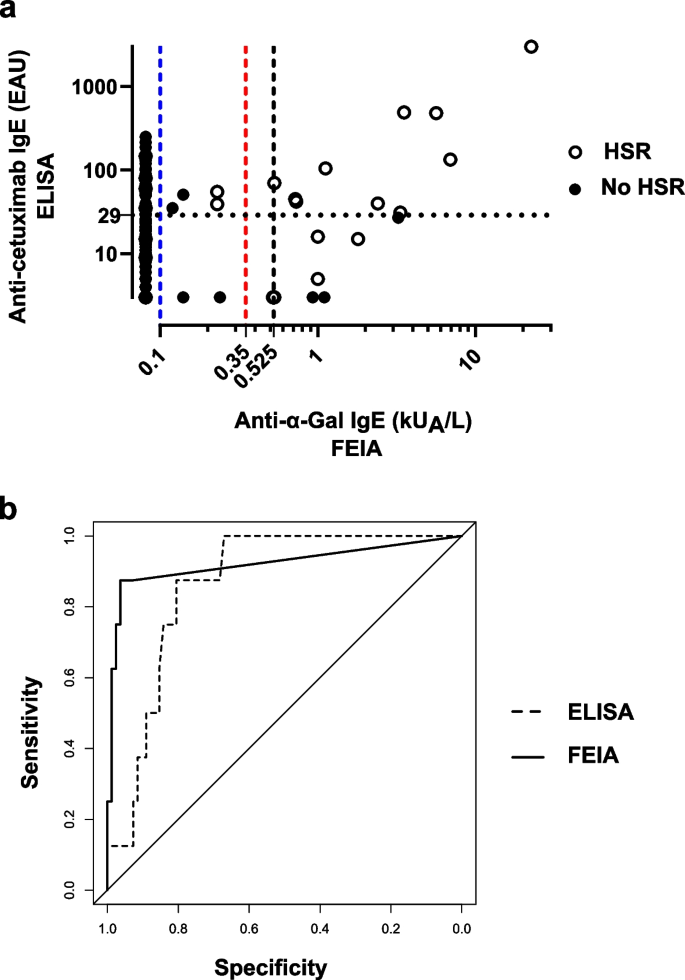
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.
Frontiers Characterization of a Diguanylate Cyclase VAGM001033 of Vibrio alginolyticus and Protective Efficacy as a Live Attenuated Vaccine Candidate in Pearl Gentian Grouper
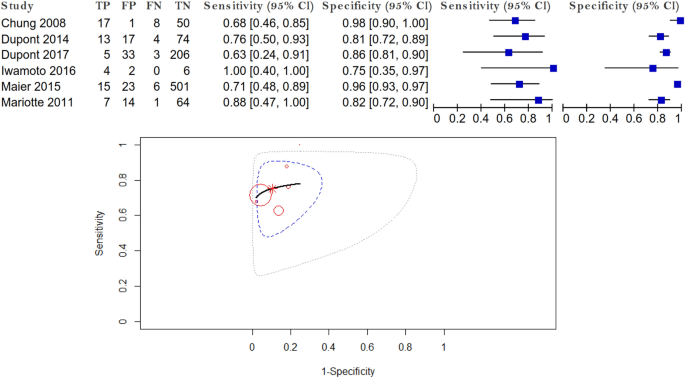
The role of IgE specific for galactose-α-1,3-galactose in predicting cetuximab induced hypersensitivity reaction: a systematic review and a diagnostic meta-analysis

PDF) Anti-cetuximab IgE ELISA for identification of patients at high risk of cetuximab-induced anaphylaxis

PDF) Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab

Prevalence of anti-cetuximab IgE. IgE levels were measured in serum

IgE to the Mammalian Oligosaccharide Galactose-α-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics—Brief Report

Allergen screening bioassays: recent developments in lab-on-a-chip and lab-on-a-disc systems
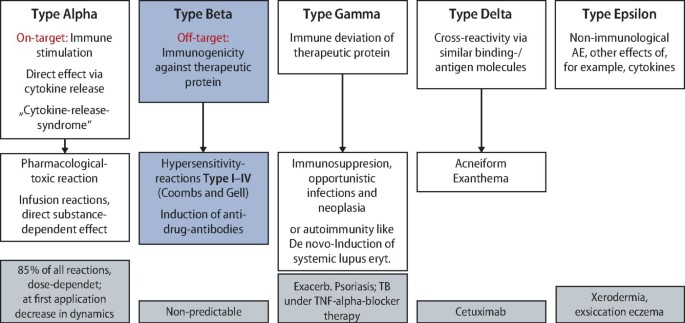
Hypersensitivity reactions to biologics (part II): classifications and current diagnostic and treatment approaches*
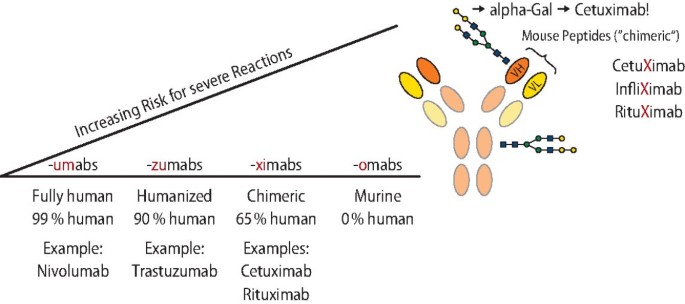
Hypersensitivity reactions to biologics (part I): allergy as an important differential diagnosis in complex immune-derived adverse events*
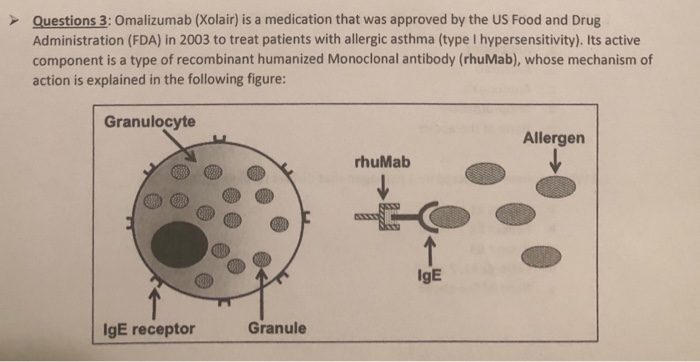
Solved Based on the information shown in the previous

Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines

(PDF) Case Report About Fatal or Near-Fatal Hypersensitivity Reactions to Cetuximab: Anticetuximab IgE as a Valuable Screening Test

PDF) Case Report About Fatal or Near-Fatal Hypersensitivity Reactions to Cetuximab: Anticetuximab IgE as a Valuable Screening Test

Anti Human IgA FITC (alpha chain specific)
Recomendado para você
-
 Boneca de pano Elsa – Frozen17 junho 2024
Boneca de pano Elsa – Frozen17 junho 2024 -
 Elsa, de Frozen: a rainha que resume todas as princesas Disney (e as mulheres)17 junho 2024
Elsa, de Frozen: a rainha que resume todas as princesas Disney (e as mulheres)17 junho 2024 -
 15 razões para as pessoas desistirem de uma vez por todas dos bolos de Frozen17 junho 2024
15 razões para as pessoas desistirem de uma vez por todas dos bolos de Frozen17 junho 2024 -
transformando princesas da disney ana|TikTok Search17 junho 2024
-
 Memetizando – Página: Array – Acabando com a sua produtividade – Blog de Humor – Tirinhas – Gifs – Prints Engraçados – Videos engraçados e memes do Brasil.17 junho 2024
Memetizando – Página: Array – Acabando com a sua produtividade – Blog de Humor – Tirinhas – Gifs – Prints Engraçados – Videos engraçados e memes do Brasil.17 junho 2024 -
 Pin em Stranger things personagens17 junho 2024
Pin em Stranger things personagens17 junho 2024 -
 Tree Buddees King Kong Climbing The Tree Funny Christmas Tree Topper - Large 10 : Home & Kitchen17 junho 2024
Tree Buddees King Kong Climbing The Tree Funny Christmas Tree Topper - Large 10 : Home & Kitchen17 junho 2024 -
 Disney's Frozen Elsa Figure17 junho 2024
Disney's Frozen Elsa Figure17 junho 2024 -
Boneca Feia em Promoção na Shopee Brasil 202317 junho 2024
-
 Chinês de cara feia - Dinheiro Rural17 junho 2024
Chinês de cara feia - Dinheiro Rural17 junho 2024
você pode gostar
-
 MIAA releases first MIAA high school hockey tournament power rankings17 junho 2024
MIAA releases first MIAA high school hockey tournament power rankings17 junho 2024 -
Cobian Sumo Terra Sandals for Men17 junho 2024
-
 Carros na Web, Volkswagen T-CROSS17 junho 2024
Carros na Web, Volkswagen T-CROSS17 junho 2024 -
 46 Kabaneri of the iron fortress ideas in 202317 junho 2024
46 Kabaneri of the iron fortress ideas in 202317 junho 2024 -
 A Máquina de Doces de Du, Dudu e Edu17 junho 2024
A Máquina de Doces de Du, Dudu e Edu17 junho 2024 -
 FIFA reconhece o Brasil como maior vencedor da Copa do Mundo Sub-17 - Confederação Brasileira de Futebol17 junho 2024
FIFA reconhece o Brasil como maior vencedor da Copa do Mundo Sub-17 - Confederação Brasileira de Futebol17 junho 2024 -
 High-Class Decks (TCG) - Bulbapedia, the community-driven Pokémon17 junho 2024
High-Class Decks (TCG) - Bulbapedia, the community-driven Pokémon17 junho 2024 -
 Full New Anime Movies in English Dubbed HD APK Download 202317 junho 2024
Full New Anime Movies in English Dubbed HD APK Download 202317 junho 2024 -
 JXK 1/6 Mini Bully Dog Model Animal American Bully Pitbull Toy Collector Decor17 junho 2024
JXK 1/6 Mini Bully Dog Model Animal American Bully Pitbull Toy Collector Decor17 junho 2024 -
Recolhedora de cafe conilon Robusta Pinhalense #robusta #agro #agrobo17 junho 2024



